The compounding industry is complex, and success requires more than just meeting minimum regulatory standards—it requires a commitment to quality, operational efficiency, and proactive planning. For 503A and 503B drug companies, partnering with a specialized consultant like Restore Health Consulting LLC can make the difference between compliance struggles and long-term success.
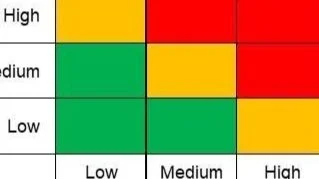
Read MoreRisk assessment is a critical component of the cleaning validation process in the compounding industry. It helps identify potential hazards, assess their impact, and prioritize efforts to mitigate risks associated with contamination. Conducting a thorough risk assessment ensures that the cleaning processes are robust, effective, and safeguard patient safety. This article describes a detailed example of how a risk assessment for cleaning validation might be conducted.
Read MoreCleaning validation is an essential process in the pharmaceutical compounding industry. Its primary goal is to ensure that compounding equipment is free of contaminants before it is used for production. This article explores the key concepts in developing a sound cleaning validation protocol.
Read MoreAs FDA has yet to finalize CGMP guidance or promulgate finalized regulations pertaining to the 503B industry, it can be challenging for 503B outsourcing facilities to fully grasp the key requirements for facility and equipment commissioning, qualification, and validation. This article aims to introduce these concepts and connect decision makers to expert CGMP facility and equipment commissioning, qualification, and validation service providers.
Read MoreA report published in 2020 by the National Academies of Science, Engineering, and Medicine (NASEM) discussing compounded bioidentical hormone replacement therapies (cBHT) could jeopardize patient access to these drugs if FDA were to follow their proposal. Since the NASEM report was released, however, a grassroots movement lead by the Alliance for Pharmacy Compounding has been mounting aimed to preserve patient access to cBHT. As of October 2, 2023, five senators have formally urged FDA to permit continued access to cBHT.
Read MoreOn September 29, 2023, updates were made to the list of bulk drug substances nominated for use under Section 503A. While GHK-Cu (with certain limitations) and L-Theanine have now been included on to the Category 1 bulks list, various peptides were added to the Category 2 bulk drug substance list (those that raise significant safety concerns).
Read MoreThe FDA released a new preliminary guidance regarding the Prohibition on Wholesaling under Section 503B of the Federal Food, Drug, and Cosmetic Act. The document aims to clarify that pharmacies licensed at the state level are permitted to dispense compounded drugs purchased from 503B outsourcing facilities. It provides specific examples of activities that are prohibited and those that are not.
Read MoreThe FDA officially withdrew the accelerated approval of Makena, a hydroxyprogesterone caproate injection, on April 6, 2023. It is suggested compounders steer clear of compounding it to remain consistent with the FDA’s sentiment. Given the lack of FDA-approved alternatives, this could prompt the clinical need for progesterone vaginal preparations as a potentially helpful alternative for this patient population.
Read MorePrescribers and regulators may question the clinical need of compounded bioidentical hormone therapies (cBHT) and how they differ from FDA approved drug products. The argument for compounding identical hormones is no longer useful, given the number of estradiol products available in topical forms, but the differences between those and what compounders offer are still unique and significant.
Read MoreFDA-approved menopausal hormone therapy (MHT) products have seen a dramatic decrease in prescribing practices over the past decade, meanwhile, custom-compounded hormone therapy (CHT) preparations have become more popular.
Read MoreThe US Food and Drug Administration (FDA) published Guidance for Industry #256: Compounding Animal Drugs from Bulk Drug Substances (GFI #256) after careful consideration and significant input from veterinarians, on August 2022. While the guidance specifically addresses compounding from bulk drug substances for animal use, several questions remain. This article aims to provide clarity to some of those questions.
Read MoreThe Food and Drug Administration (FDA) added amoxicillin oral powder for suspension to the drug shortage list and has released guidelines on the creation of compounded versions of specific beta-lactam oral suspension products due to the current lack of amoxicillin oral antibiotic powder for suspension. High demand for amoxicillin oral antibiotic suspension products, coupled with requests for clarification on the preparation of compounded versions, have prompted the Federal Agency to issue compounding recommendations for pharmacists in state-licensed pharmacies and federal facilities not registered as outsourcing sites.
Read MoreAs alluded in a recent declaration that desiccated thyroid extract (DTE) is considered a biologic, FDA may be in the beginning stages of banning compounded desiccated thyroid extract. Compounding pharmacies believe the announcement is a direct threat to the future of compounded thyroid drugs. What remains to be seen is how quickly (or if ever) the federal agency will move to enforce this position. To gauge the future of compounded drug products, one must ask is thyroglobulin considered an inactive ingredient or a biologic?
Read MoreOn November 1, 2022 the United States Pharmacopeia (USP) published the final revision to General Chapter <797> Pharmaceutical Compounding of Sterile Preparations. Compounding pharmacies must clearly understand how the new standards affect their compounding operations and take proper steps to comply. Changes become official on Wednesday November 1, 2023.
Read MorePharmacy compounding is a crucial aspect of the pharmaceutical industry that involves the preparation of customized medications for individual patients based on their specific needs. The compounding process involves various steps, starting from the selection of appropriate raw materials, to the final dispensing of the compounded medication. To ensure the quality and safety of compounded medications, compounding standards have been established by the United States Pharmacopeia (USP). In recent years, regulatory agencies including state boards of pharmacy and FDA have placed increased emphasis on the enhanced regulation of compounding practices.
Read MoreThe information below represents material from the conference and includes data from the 503B Market Landscape Study conducted by the FDA in 2022. These points include emerging compounding trends and key industry insights shared by FDA.
Read More503B outsourcing facilities are required to report adverse events to FDA to stay compliant. 503Bs have noticed an upward trend in audits surrounding adverse events, citing that state boards and the FDA believe adverse events are not being reported properly. This article summarizes 503B responsibilities in reporting adverse events to FDA.
Read MoreCompounding pharmacy business owners find that job ads posted on online employment websites like Indeed and LinkedIn aren't cutting it in today's challenging job market. Even recruiters can't seem to fill positions. Employers are having trouble attracting new employees as well as retaining the talent they have while pharmacy employees lament they want more out of their workplace — a sense of purpose, work/life balance, recognition, appreciation, and advancement opportunities.
Read MoreOn July 22, 2022, the FDA proposed a new rule to update the NDC, or National Drug Code, format from a 10-digit code to a 12-digit code over the next five years citing that the NDC will eventually run out of 5-digit labeler codes within the next 10 to 15 years.
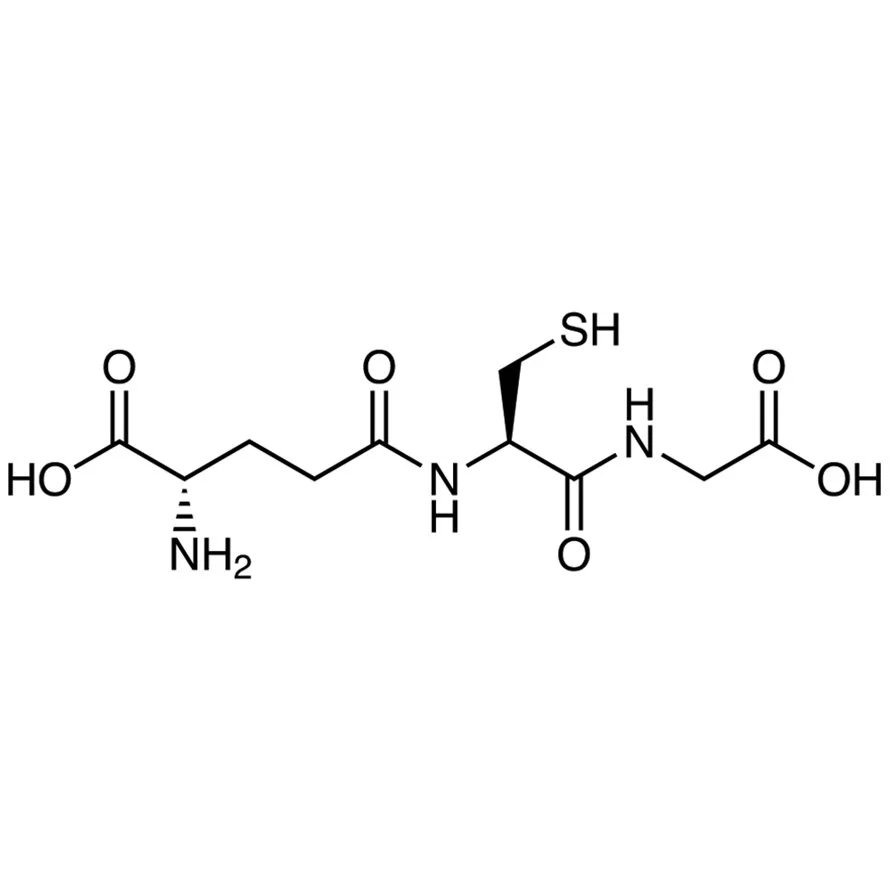
Read MoreOn June 8th 2022, the FDA’s Pharmacy Compounding Advisory Committee, or PCAC, voted 8-5 with one abstention to add glutathione to the final 503A Bulk Drug Substance List. In its evaluation, FDA found there to be a lack of effectiveness and safety data of glutathione and recommended against its inclusion onto the final 503A Bulks List. While PCAC rarely votes against FDA’s direction, that is exactly what happened in this instance.
Read More