503A vs. 503B Compounding & FDA Regulations Guide
Following increased regulatory scrutiny and safety incidents linked to inconsistent oversight by state boards of pharmacy, the FDA established two designations for compounding facilities: 503A and 503B. This guide explains the defining features of each designation, the key differences between them, and what 503A compounding pharmacies and 503B outsourcing facilities need to know to remain compliant with evolving FDA and state regulations.
Key Takeaways
If you’re short on time, here’s a quick summary to understand the key differences between 503As and 503Bs right away:
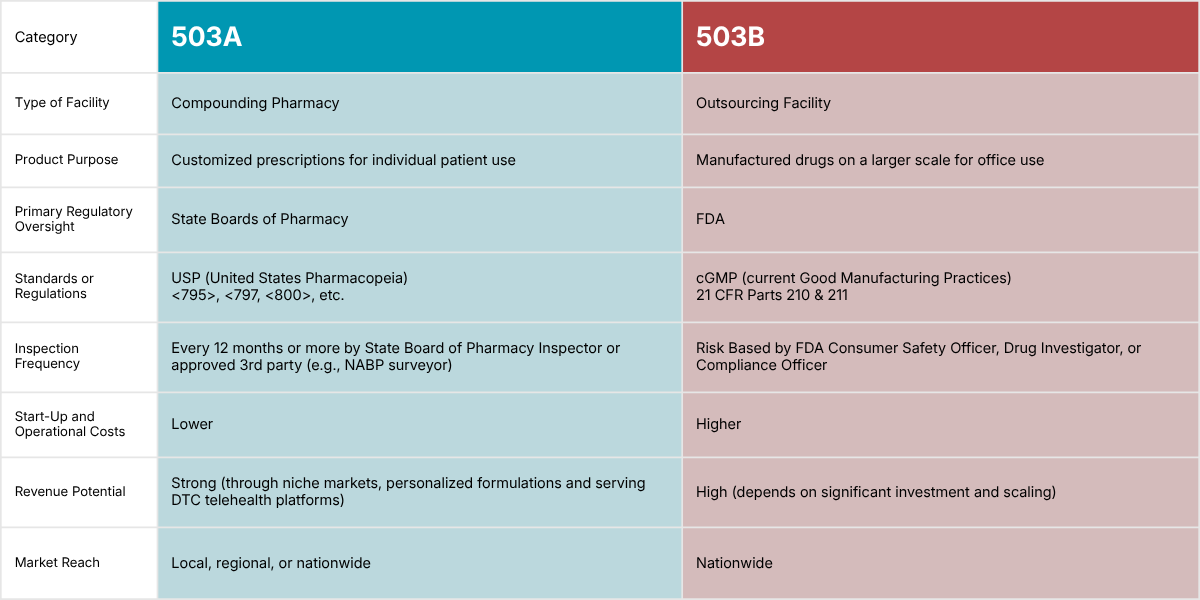
503A compounding pharmacies compound medications according to individual patient prescriptions, which may be intended for either at-home use or administration in a healthcare setting, while 503B outsourcing facilities compound drugs in larger batch sizes under cGMP with or without patient-specific prescriptions to be distributed (generally) to healthcare facilities for office use.
503A compounding pharmacies must comply with applicable state board of pharmacy regulations and USP standards (e.g., <795>, <797, <800>). Beyond Use Dates (BUD) are assigned to each compound based on USP upper limits, supported by stability studies or general scientific literature reference.
503B outsourcing facilities are primarily regulated by the U.S. Food and Drug Administration and must comply with federal regulations (namely 21 CFR Part 210 and 211), state regulations applicable to 503Bs, and current good manufacturing practice (CGMP). BUDs or expiration dates are assigned to each compound and are based on validated scientific methods and testing of the specific product under controlled conditions.
Key difference: 503Bs must comply with federal current good manufacturing practice (CGMP), which includes validating or qualifying critical systems, equipment, and processes as applicable, while 503As must comply with specific state regulations and minimum industry standards.
At Restore Health Consulting, we specialize in supporting 503A and 503B compounding facilities. We don’t believe in one-size-fits-all consulting solutions, and we’re not a general consulting firm. Our services are designed and tailored to help you build, grow, and restore your business to operational excellence.
Investing in specialized consulting is critical for compounding entities, and you need someone who understands your unique challenges and regulatory requirements. We want to be your go-to partner for achieving sustainable success, so don’t hesitate to book a free consultation with one of our experts today.
Read more about why 503As and 503Bs need advisors and how we can help in this article.
503A vs. 503B Overview
Both 503A and 503B facilities are critical for healthcare providers, pharmacists, and patients alike. Compounding facilities play an essential role in providing customized medications for patients with unique needs. They also serve an important supporting function in the U.S. healthcare supply chain.
However, 503A compounding pharmacies and 503B outsourcing facilities don’t operate under the same regulatory framework. They also differ in their scale of production (e.g., 503As are limited to 250 sterile compounded units per batch vs. 503Bs have no limit on batch size) and the intended use (e.g., at home vs. in-office) of the medications they produce.
To put it simply, what we associate with a "traditional compounding pharmacy" is a 503A pharmacy. These pharmacies prepare medications as per the direction of an individual patient prescription, are generally intended for home use or administration in a healthcare setting, and sterile preparations cannot be produced in excess of 250 units per batch.
In contrast, a 503B outsourcing facility is an FDA-registered entity that compounds large volumes of sterile drugs for healthcare providers. It sits somewhere between a traditional pharmaceutical manufacturer and a compounding pharmacy, but with its own distinct regulatory framework (including FDA inspections and adherence to cGMP).
The reality is complex: compounding regulations are stringent for a reason, ensuring patient safety at every level. Understanding each facility’s regulatory framework is critical for compliance and business success.
Which Act Clearly Describes the Difference Between 503A and 503B?
The distinction between 503A and 503B compounding facilities is outlined in the Federal Food, Drug, and Cosmetic Act (FD&C Act), as amended by the Drug Quality and Security Act (DQSA) of 2013.
What is the FDA 503A and 503B?
The FDA 503A and 503B refers to sections of the Federal Food, Drug, and Cosmetic Act (FD&C Act), which created two separate regulatory pathways for compounded medications:
“503A” comes from Section 503A - Pharmacy Compounding of the FD&C Act, and it defines the 503A compounding pharmacy pathway
“503B” comes from Section 503B - Outsourcing Facilities of the FD&C Act and it defines the 503B outsourcing facility pathway
Section 503B was established following the 2012 New England Compounding Center (NECC) tragedy, a nationwide fungal meningitis outbreak leading to over 50 deaths, caused by contaminated injectable steroid medications. It led the congress to sign the Drug Quality and Security Act (DQSA), which amended the FD&C Act and gave birth to the 503B outsourcing facility designation.
This helped establish outsourcing facilities as a new category of compounders. FDA oversight of large-scale sterile drug compounding strengthened and aimed to enhance product safety and quality. It also allowed 503Bs to provide compounded drug products that are not otherwise commercially available.
503A Compounding Pharmacies
503A pharmacies are compounding pharmacies that prepare medications based on individual patient prescriptions for home use.
Benefits and Advantages
Governed primarily by state boards of pharmacy rather than federal manufacturing regulations
Typically meet minimum industry standards set by USP
Overall lower regulatory risk compared to 503B
May have a greater number of formulation offerings due to lower development costs, allowing for a wider variety of dosing options and formulations
Lower initial startup cost compared to starting a 503B
Lower operational requirements compared to 503Bs
Faster time to market for new compounding services
Lower annual fees (primarily state licensing fees, compared to significant annual FDA establishment and potential reinspection fees for 503Bs)
Can focus on developing a local business or scaling and targeting the broader market
Personalized patient care, tailored formulations for specialized treatment plans and patient needs, consultation opportunities
Significant growth and scale-up opportunities
Strong revenue potential through niche markets and personalized formulations
Challenges and Disadvantages
Can’t produce batches of CSPs in excess of 250 units
Can supply compounded medications only under patient-specific conditions
Patients often have to cover medication costs out-of-pocket
Not subject to the same batch test release requirements under federal cGMP, but expected to ensure quality through USP- and/or state board of pharmacy-compliant procedures and documentation
Can a 503A Pharmacy Purchase Products Compounded by a 503B Facility?
Yes. A 503A pharmacy may purchase compounded drugs from a 503B outsourcing facility for patient-specific dispensing, subject to the FDA’s 2023 FDA draft guidance and applicable state laws.
The 2023 draft guidance is a standalone document outlining FDA’s current thinking on the prohibition on wholesaling under Section 503B, separate from the temporary COVID-19 policies that have since been withdrawn. By leveraging the manufacturing capabilities of 503B facilities, 503A pharmacies can now expand their compounding services and ensure a steady supply of necessary medications for their patients. Note that while the guidance reflects current FDA thinking at the federal level, certain states have taken issue with this model and have prohibited the practice in recent years.
Pharmacies that purchase 503B-manufactured compounds must also be vigilant in ensuring that they do not exceed the scope of the federal guidance. For example, a 503A cannot resell a 503B-sourced compounded drug to a healthcare provider for office use. This means that the compounded medication is not allowed to be wholesaled by the pharmacy, or transferred to another pharmacy or physician’s office, even for dispensing to an individual patient.
Instead, the 503A is limited to dispensing the 503B product only pursuant to a valid prescription directly to the identified individual patient. Any deviation from this could result in regulatory action from both state boards of pharmacy and the FDA.
The guidance also outlines that the 503A may not further compound or materially manipulate a finished 503B product, though applying the pharmacy’s dispensing label is permitted. However, this facet of the FDA guidance may conflict with certain state regulations.
If you need help navigating regulations in your state and how they apply to your business, don’t hesitate to schedule a free consultation with one of our consultants.
503B Outsourcing Facilities
A 503B outsourcing facility is a type of drug manufacturing establishment authorized to compound medications at significantly larger scale than a traditional 503A pharmacy, with or without patient-specific prescriptions, for distribution to healthcare facilities for office or “office-administered” use.
Although the term “503B pharmacy” is sometimes used online, these establishments are officially designated as 503B outsourcing facilities — often referred to simply as 503B facilities or 503Bs.
Read More: What is a 503B? Outsourcing Facilities Explained
Benefits and Advantages
Produce large batches and compound sterile drugs
Can compound without individual prescriptions
May maintain larger inventories for anticipated demand
Costs to patients may be lower for some 503B-compounded products, depending on the product and market conditions
Preparations have to be tested for potency, integrity of label claims, and stability
Nationwide distribution allowed
May create direct supply relationships with institutions like hospitals
Greater operational flexibility, with a potential to serve broader markets
Greater growth and scaling opportunities compared to 503As
Premium pricing opportunities during drug shortages
High revenue potential that depends on significant investment and scaling
Challenges and Risks
More stringent and complex regulations and overall higher regulatory risk compared to 503As
Are limited in terms of which bulk drug substances can be used in compounding
Cannot compound copies of commercially available FDA-approved drugs (Check out these FAQs)
Due to high development costs, may need to start with a small number of SKUs (Stock Keeping Units)
Higher initial startup cost compared to launching a 503A
Higher operational requirements compared to 503As (facility, equipment, materials, processes, and personnel)
Must be purpose-built to handle sterile compounding operations and meet the stringent requirements outlined in cGMP regulations
Must have a robust quality system that encompasses management oversight, risk assessment, and continuous improvement
Higher annual fees (annual FDA registration and listing fees, potential reinspection fees)
Higher business risk
Regular FDA inspections and potential enforcement actions
Can a 503B sell to a 503A?
The 2023 FDA draft guidance, issued to address public health needs, permits 503B facilities to sell compounded drugs to 503A pharmacies, which can then dispense these products directly to patients pursuant to a valid prescription.
While the FDA draft guidance permits the sale of 503B compounded drugs to 503A pharmacies, these pharmacies remain responsible for adhering to all relevant state laws regarding the receipt, storage, and dispensing of these medications. This includes maintaining proper documentation for each prescription, ensuring the quality and safety of the compounded drugs, and adhering to state-specific compounding regulations.
It’s also important to note that in recent years, certain states specifically prohibited this type of sales channel (e.g., Massachusetts, Idaho, Mississippi, etc.). Other states may accept this model but explicitly require the 503B be licensed in their state in order to ship those medications into their state (e.g., California, etc.).
503B facilities may not engage in wholesale distribution of the compounds produced. This means they’re not allowed to sell to buyers who will resell.
Read More: Considerations for the 503B-to-503A-to-Patient Compounding Model
Key Differences Between 503A and 503B: Regulations & Guidelines
503A
Have to be registered with the Drug Enforcement Administration (DEA) and as a manufacturer of controlled substances (if manufacturing and distributing controlled substances)
Have to meet state pharmacy board regulations and pertinent federal laws (section 503A of FD&C Act)
Have to comply with USP <795>, <797>, and <800> as applicable
Not required to comply with cGMP
Don’t need to be registered with the FDA
Facility certifications are required every six months
Beyond Use Dating (BUD) is typically shorter per USP limits and may be assigned based on internal or external scientific scientific evidence, not necessarily on product-specific stability testing
503B
Regulated primarily by the U.S. Food and Drug Administration (FDA)
Must register with the FDA
Have to meet applicable state regulations in states they are licensed in
Required to adhere to 21 CFR Part 210 and 211 under current Good Manufacturing Practices (cGMP)
Facility, utilities, and equipment must be validated at start-up and routinely re-validated
Environmental monitoring programs in 503B settings are typically more extensive and frequent than those required for 503A sterile compounding, reflecting cGMP expectations.
Must maintain their own quality department
Must report their product list to FDA biannually
Beyond Use Dating (BUD) is longer and more consistent, based on scientific testing of the specific product under controlled conditions
Failing to meet these standards can lead to FDA warning letters, product seizures, or even facility shutdowns. Compliance violations not only lead to immediate financial penalties but also result in long-term damage to your standing with regulators, making it more difficult for the facility to maintain compliance with FDA and state regulatory requirements.
If you’re looking for in-depth information about starting a 503B outsourcing facility, read this article. If preparing for a 503B outsourcing facility FDA inspection, access this guide.
cGMP Breakdown
The major difference between 503As and 503Bs when it comes to drug manufacturing processes is that 503Bs have to comply with Current Good Manufacturing Practices (cGMP), while 503As don’t.
Current Good Manufacturing Practices (cGMPs) are the backbone of any successful 503B outsourcing facility. These practices are designed to ensure the consistent production of safe and high-quality products.
Key cGMP Considerations:
Written Standard Operating Procedures (SOPs): Every aspect of sterile drug production must be documented in SOPs, including compounding processes, cleaning procedures, and quality control measures.
Environmental Monitoring: Regular monitoring of the cleanroom environment for airborne particles and microbial contamination is required to ensure that the environment meets ISO standards. This includes the use of settle plates, active nonviable and viable air samplers, and surface swabs to detect contaminants.
Validated Processes: All systems (e.g., facility, equipment, process, materials, personnel) must be validated or qualified to ensure they consistently produce quality products.
Batch Records: Detailed records must be kept for each batch of compounded drugs, including information on raw materials, equipment used, processing steps, and final product testing.
Quality Control and Testing: Before a product is released, it must undergo rigorous testing to ensure it meets predefined quality specifications. This may include but is not limited to sterility testing, pyrogen (endotoxin) testing, and potency testing as applicable.
Ensuring that the facility adheres to cGMP from day one is critical, as the FDA will regularly inspect 503B facilities for compliance with these standards.
Read about these processes and what you need to know if you’re starting a 503B Outsourcing Facility here.
In Conclusion
Whether operating as a 503A compounding pharmacy or a 503B outsourcing facility, understanding and maintaining compliance with FDA and state regulations is critical to ensuring patient safety, operational efficiency, and long-term business success.
Both 503As and 503Bs serve vital but distinct roles in the healthcare ecosystem. 503As advance personalized medicine and patient care, and 503Bs ensure large-scale, quality-controlled access to sterile medications.
Knowing where your facility fits within this framework helps you make informed decisions about investment, staffing, and growth strategies.
At Restore Health Consulting, we specialize in supporting 503A and 503B compounding facilities through every stage of their business journey—from setup and licensing to inspection readiness and operational excellence. Our consulting services are tailored, not templated, because no two facilities face the same challenges.
If you’re ready to strengthen your compliance strategy or explore new growth opportunities, book a free consultation with one of our experts today. We’ll help you stay compliant, future-proof your operations, and position your business for sustainable success.